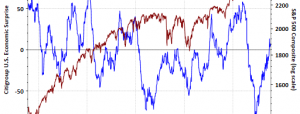
When it rains it pours for Mallinckrodt (MNK). Last month a Delaware judge invalidated 11 patents pursuant to Inomax, one of the company’s top-selling treatments; it could pave the way for Praxair (PX) to offer a generic version. The Journal of the American Medical Association (“JAMA”) followed that up by questioning the effectiveness of Acthar for certain indications:
Over the past five years, why has the US government spent $1 billion on a drug that is no more effective than alternatives that are tens of thousands of dollars cheaper per treatment?
The drug is called Acthar, and for the past year it has been the focus of a study by the Oregon Health and Science University School of Medicine and Oregon State that has been trying to understand why doctors keep prescribing it for ailments it has never been proved to treat effectively.
At 39% of total revenue, Acthar is Mallinckrodt’s largest-selling product. Acthar and Inomax represent a combined 54% of revenue, so over half the company’s revenue could have question marks surrounding it.
Did JAMA Damage The “Acthar Effectiveness” Argument?
Acthar treats infantile spasms and certain autoimmune conditions. Its Q2 revenue grew 7% Y/Y, compared to a 5% decline for the entire company. Mallinckrodt grew its operations through acquisitions; in an about face it recently began to divest properties in to order to pare its $5.9 billion debt load. Over the past year the company engaged in asset sales of over $800 million. It is now more dependent upon Acthar whose revenue was only 31% of the company’s total in the second half of 2016.
In 2015 Citron’s Andrew Left questioned the effectiveness of Acthar and contended Mallinckrodt could have problems with reimbursements from insurance companies. Management is currently providing studies to prove Acthar’s effectiveness, yet no conclusions have been reached:
We were also pleased that four new Acthar papers were published in the last several months, which we believe provide additional evidence for payers and prescribers to support the positioning of the product, primarily for refractory patients. Three of the publications report on clinical experience for Acthar in key promoted indications: rheumatoid arthritis, nephrotic syndrome and sarcoidosis, with clinical studies in these patient populations providing further evidence to support the treatment of refractory patients who can benefit from Acthar.
The fourth is a recently published article summarizing the extensive data contained in our Academy of Managed Care Pharmacy, or AMCP, dossier for Acthar … Primarily used with managed care organizations, the dossier contains data on proper use for appropriate patients and the health economic benefits of the drug.