At first Aclaris [Nasdaq: ACRS] didn’t catch my eye. The phrase “concentrated hydrogen peroxide” sounded too simple to offer any sustainable competitive advantage.
However a favorable mention from the famous “Dr. KSS” who knows his biotechnology made me take a closer look. What I see is a fairly low-risk to treat a very common skin condition (Seborrheic Keratosis “SK”) and a reasonable chance to expand into common warts and Alopecia Areata.
Their A-101 drug for SK is applied topically which is a major advantage. The Phase 2 results for SK show great effectiveness and create what may be a very low risk Phase 3 trial during 2016 with an NDA filing target of 4Q 2016. That would mean a commercial launch into a fairly large dermatological market in 2017.
SK is very common and there are already 8.3 million procedures per year. Current options are however *not* topical and include cryosurgery, excision, and curettage which are much less friendly. It’s logical to expect that a greater portion of the 18 million visits to a dermatologist for this condition would agree to treatment if the topical option was available. Some portion of the existing treatments would probably flip to the topical option as well.
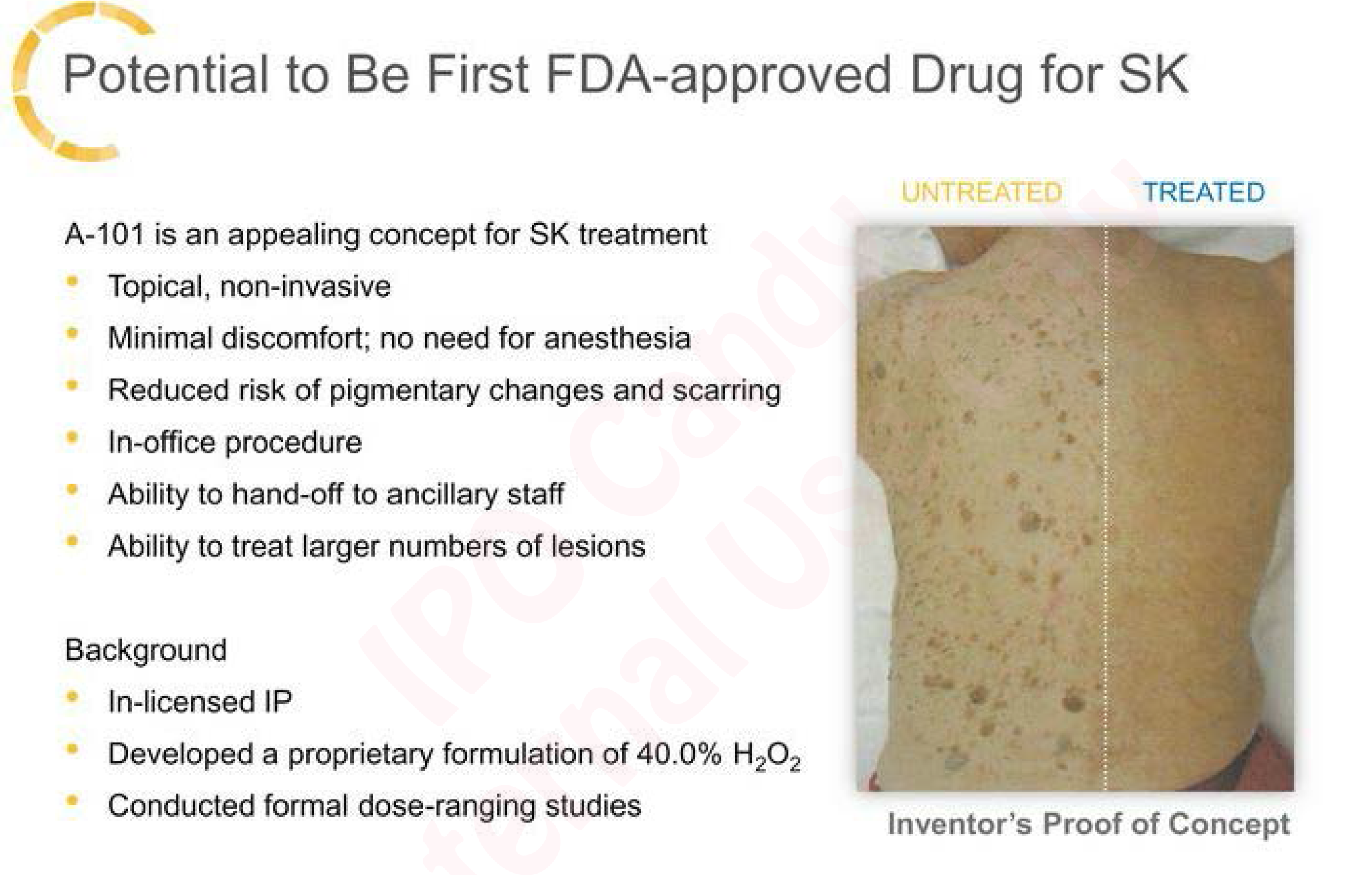
Putting a figure on market size we’d estimate that half of the 10 million who decide not to seek treatment would opt in and half of the existing treatment market would flip to topical. Using average net revenue of $100/treatment for the “new” patents and $200/treatment for the “flip” patients (because they typically would have more serious conditions) we get to estimated annual market of $1.3B for topical out of what would be a $2B overall market. This would expand by another $100M to $200M upon approval for the treatment of common warts.
Aclaris has in-licensed two compounds (A201 and A301) that appear promising for treating Alopecia Areata but it’s further out – IND planned for 2H2016 with a POC trial in 2017. If one were to factor that in the market opportunity is sizable with an estimated 6.6 million cases in the US and there is no treatment besides off-label use of steroids. If half of the patients diagnosed took treatment and we use a $500 net price we’d get to an additional $1.6B. It’s too early to put a finer point on it but it’s considerable.