Share markets in India are presently trading marginally higher. Sectoral indices are trading on a positive note with stocks in the banking sector, metal sector and telecom sector witnessing maximum buying interest.
The BSE Sensex is trading up by 157 points (up 0.4%), while the NSE Nifty is trading up by 34 points (up 0.3%). The BSE Mid Cap index is trading up by 0.4%, while the BSE Small Cap index is trading up by 0.6%.
The rupee is trading at 68.68 to the US$.
In the news from pharma space, Alembic Pharmaceuticals share price is in focus today. This comes as the company has received tentative approval from the US Food and Drug Administration (USFDA) for Bimatoprost Ophthalmic Solution, used to treat hypotrichosis of the eyelashes by increasing their growth including length, thickness and darkness.
As per the company filing, the tentatively approved Abbreviated New Drug Application (ANDA) is therapeutically equivalent to the reference listed drug product (RLQ), LATISSE ophthalmic solution, 0.03% of Allergan.
At the time of writing, Alembic Pharmaceuticals share price was trading up by 1.6% on the BSE. The drug had an estimated market size of US$ 63 million for 12 months ending December 2017.
Indian pharma companies catering to the US markets are breathing a sigh of relief. After being adversely affected by import bans and the suspension of new drug approvals from manufacturing facilities in the past three years, there has been a sharp pick-up in new drug approvals in FY17.
With an aim to lower the overall health care costs in the country, the US Food and Drug Administration (FDA) approved a record 763 generic drugs for the financial year ending 30th September. As per Mint Analysis, Indian pharma companies received 295 approvals accounting for 40% of the overall approvals during the year.
Generic Drug Approvals Hit the Roof
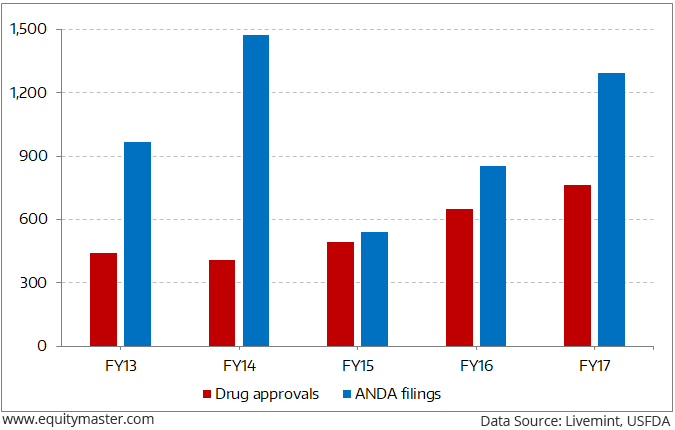
Even the total filings of abbreviated new drug applications (ANDAs) for generic drugs rose to 1,292 in FY17 from 852 in FY16. While faster approvals expedite the commercialization of product pipelines of domestic pharma companies spurring growth, at the same time, however, it has raised the intensity of competition resulting in pricing pressures. The price erosion has been further compounded by a consolidation among US distributors and the decline in the number of products going off-patent over the past few years.